
2020-01-17 Views:4375
Warm Tip: If you want to know more information, like quotation, products, solutions, etc., please Click here ,and contact us online.

Cyanide is widely used in gold leaching industry in the past years, it is cheap and suitable for leaching process. However, cyanide has its own weaknesses, it is high-poisonous and bad to environment and human being. Many countries in the world have banned the using of cyanide. But many eco-friendly gold leaching reagent appeared since then. For example, CNLITE gold leaching reagent. It can replace cyanide in gold leaching plant without changing the original process and equipment, and it’s eco-friendly and low-poisonous. It’s a good choice for gold leaching plants.
Cyanide is an essential agent for gold extraction by cyanidation. Commonly used cyanide agents are NaCN, KCN, NH4CN, CaCN2 and cyanide melts. The cyanide melt is a cheap cyanide, the useful component is 45% CaCN2, and the rest are impurities such as soluble sulfide, carbon and insoluble matter. Before using, the sulfide is vulcanized by vigorously stirring the solution and adding lead salt to it. Lead precipitation, clarified solution for cyanidation.
When selecting cyanide, consider the relative solubility of cyanide to gold, stability, impact of impurities on the process, price and reliability of supply. The relative consumption of cyanide for equal solubility: KCN>NaCN>Ca(CN)2> NH4CN; cyanide can be decomposed into HCN in air containing carbon dioxide, its stability: KCN> NaCN > NH4CN> Ca(CN )2.
The most commonly used in industrial production is Sodium cyanide. It should be pointed out that according to the basic reaction formula of cyanide-dissolving gold, the theoretical dissolution of 1 gram of gold requires only 0.49 g of Sodium cyanide, but in actual production, the actual consumption is more than 200 times the theoretical consumption due to mechanical and chemical losses.
With the increasingly prominent contradictions between economic benefits and environmental protection, the requirements for the development of non-toxic and environmentally-friendly gold-dressing reagents as alternatives to cyanide for leaching gold ores are becoming more and more urgent. Research and development of non-toxic gold-dressing reagents with high performance and high market prospects plays an important role in the development of the gold industry.
In order to solve the problem of highly toxic pollution of cyanide, for decades, scholars at home and abroad have carried out a large number of experimental research work, and strive to find a cyanide-free, non-toxic or low-toxic beneficiation reagent as alternatives to cyanide. Finally, several leaching reagent had been researched, such as: thiourea, water chlorine, coal agglomeration, thiosulfate, stone sulfur, hypochlorite, etc., but for various reasons these alternatives to cyanide have not achieved industrial production and use.
Among them, thiosulfate is considered to be the most promising gold leaching reagent as alternatives to cyanide because of its rapidity and non-toxicity. However, when there is only thiosulfate in the immersion gold solution, the gold dissolution is very slow.
In order to increase the leaching speed, the researcher adds copper (II) ions to the thiosulfate solution, and the ammonia water is used as a mixed solution as a gold extraction leaching solution. In the presence of copper ammine ions, although the gold dissolution rate is increased, the consumption of thiosulfate is sharply increased, and the products of the thiosulfate are numerous, which makes the gold leaching composition complicated, and the gold entering the leaching solution is difficult to be effectively recovered. With the defects of large consumption, the thiosulfate reagent is difficult to industrialize production and use.
With the call of the times, CNLITE eco-friendly gold ore dressing reagent that can be completely alternatives to the highly toxic sodium cyanide in gold leaching process appeared. 100% environmental protection. 100% safety.
Under other same leaching conditions, the economic benefit analysis of comparable items was carried out for the amount, leaching rate and gold adsorption rate. The comparison test results prove that CNLITE eco-friendly gold leaching reagent almost obtain the equal economic benefit for the gold mine as sodium cyanide when used as leaching agents. From the perspective of environmental protection and safety, CNLITE is a perfect alternatives to cyanide for leaching gold ores.
In order to investigate the difference of sodium cyanide leaching reagent and CNLITE leaching reagent in the leaching effect on gold ore, we choose six kinds of gold ore samples which are oxidized gold ore in Sultan Kush, oxidized gold ore in Tanzania , oxidized ore and mixed ore of gold mine in Tanzania , oxidized gold ore gravity separation tailings in Tanzania (T5) for leaching comparison test.
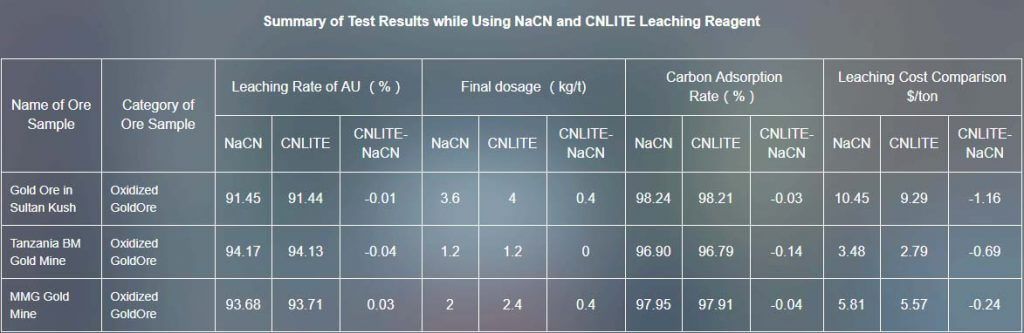
The leaching rate of CNLITE leaching reagent is nearly same as sodium cyanide leaching reagent, while the cost of leaching is lower. Besides, CNLITE leaching reagent is eco-friendly and low-poisonous, while the sodium cyanide leaching reagent is bad to environment. The greater thing is, when using CNLITE leaching reagent, you don’t have to change the original process and equipment, which would not cause any additional cost.
In conclusion, in order to make the leaching plant more eco-friendly, changing cyanide to CNLITE gold leaching reagent is a great choice.
 86 18716000713
86 18716000713
 shirleyyin321@gmail.com
shirleyyin321@gmail.com
 No. 188, Xinhai Street, high-tech Industrial Park, Fushan District, Yantai, Shandong, China.
No. 188, Xinhai Street, high-tech Industrial Park, Fushan District, Yantai, Shandong, China.
